CINÉTICA ENZIMATICA (ecuación de michaelis Km)
Cinética en Saint Marc: Enzimas y Velocidad de Reacción
In this section, the discussion revolves around enzyme kinetics in Saint Marc, focusing on the models of enzyme-substrate interactions and the concept of enzymatics in biochemistry.
Models of Enzyme-Substrate Interactions
- The Fisher model suggests a perfect fit between substrate and enzyme, akin to a lock and key.
- Conversely, the Koshland model proposes an induced fit mechanism where the substrate alters the enzyme for binding.
Mechanisms of Enzyme Action
- Enzymes operate through various mechanisms:
- Proximity mechanism requires substrates to approach each other closely for reaction.
- Acid-base mechanism involves pH-dependent reactions where enzymes deform substrates to break bonds.
- Covalent mechanism forms temporary covalent bonds between enzyme and substrate during production.
Understanding Enzymatics
- Enzymatics studies enzymatic reaction rates and factors affecting them, primarily influenced by substrate concentration.
- The substrate velocity curve illustrates that higher substrate concentrations lead to increased reaction rates until reaching a maximum velocity.
Significance of Constants in Enzyme Kinetics
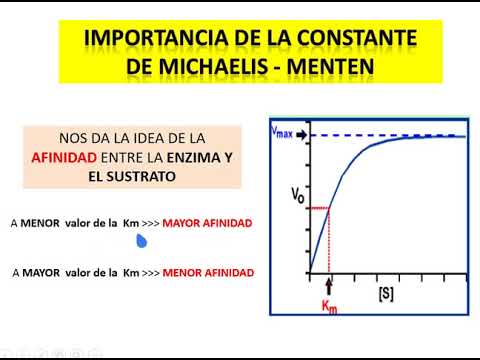
- The Michaelis constant (Km) indicates half-maximal velocity substrate concentration, crucial for understanding reaction dynamics.
- High substrate affinity results in rapid saturation and lower Km values, impacting reaction rates significantly.
Importance of Affinity in Enzyme Substrate Interactions
This segment delves into the significance of affinity between enzymes and substrates, elucidating how it influences reaction rates and constants.
Affinity Determination
- Michelis constant reflects affinity levels; higher affinity corresponds to lower constants indicating faster saturation.
State Steady Complex Formation
- The formation of an enzyme-substrate complex at steady state determines reaction velocities accurately.
Reaction Rate Formulas
- Reaction rate equations establish relationships between velocity, substrates, and constants for varying concentrations.
Afinidad enzimática y reacciones enzimáticas
In this section, the discussion revolves around enzyme affinity and enzymatic reactions, exploring how factors like temperature, pH, and substrate concentration influence reaction rates.
Enzyme Affinity and Enzymatic Reactions
- Enzymes with high affinity quickly saturate their enzymes when interacting with substrates, leading to vasodilation due to acetaldehyde accumulation in excessive alcohol consumption.
- Affinity in enzymes is crucial for reactions. The example of a linear graph conversion from the Michaelis-Menten curve illustrates this importance.
- Factors influencing enzymatic reactions include temperature (optimal or slightly elevated temperatures increase substrate movement and reaction speed), optimal pH (around 5.9 enhances reaction rate), and other components.
- Substrates reach maximum velocity when implemented. An enzymatic reaction demonstration shows how increased substrate molecules elevate production speed significantly.
- Increasing substrate molecules further boosts production speed until enzyme saturation occurs, limiting the reaction rate's increase.
Influence of Temperature and pH on Enzymatic Reactions
- Elevated temperatures enhance substrate movement and product formation but can lead to enzyme denaturation beyond a certain point (around 75 degrees).
- Similarly, pH affects reaction rates; while moderate changes may improve reactions, extreme pH levels can denature enzymes or slow down reactions significantly.
Inhibición enzimática
This part delves into enzymatic inhibition types - competitive, non-competitive, and uncompetitive - impacting enzymatic reactions differently based on inhibitor interactions with active sites.
Types of Enzymatic Inhibition
- Enzyme inhibition involves factors that hinder enzymatic reactions. It categorizes inhibitors as non-specific irreversible inhibitors or specific reversible inhibitors like competitive, non-competitive, and uncompetitive inhibitors.
- Competitive inhibition occurs when an inhibitor competes with the substrate for the active site. This competition alters enzyme-substrate binding dynamics affecting reaction rates.
- Non-competitive inhibition involves inhibitors binding to sites other than the active site. This alters enzyme shape hindering substrate binding without changing affinity but reducing reaction speed.
Impact of Inhibition on Reaction Rates
- Uncompetitive inhibition occurs post-substrate binding at a different site altering enzyme conformation. While affinity remains constant, reduced product formation decreases reaction rate.
- Competitive inhibition reduces both constants in the equation affecting initial substrate concentration values. Understanding these inhibitions aids in pharmacology for effective drug design based on their mechanisms.
Conclusion